DESCRIPTION
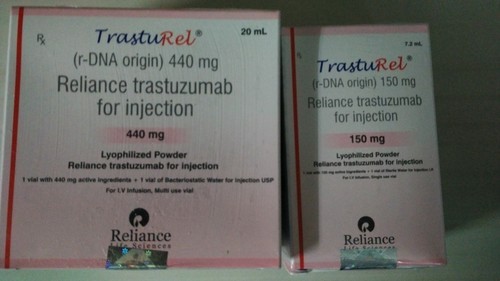
TrastuRel (Trastuzumab) is a monoclonal antibody that is specifically designed to target HER2 receptors on breast cancer cells. It flags the cancer cells for destruction by the body’s immune system. TrastuRel is injected into a vein through an IV. It is a prescription drug.
Manufacturer : Reliance Life Science
ADDITIONAL INFORMATION
Strengths available : 150mg & 440mg
Storage : Store at temperature between 2°C – 8°C.
Dosage :
Precautionary Measures :
- Do not administer as an intravenous push or bolus.
- Do not mix Trasturel with other drugs.
- Do not substitute Trasturel (Trasturel) for or with ado-Trasturel emtansine.
Recommended Doses and Schedules:
Adjuvant Treatment, Breast Cancer: Administer according to one of the following doses and schedules for a total of 52 weeks of Trasturel therapy:
During and following paclitaxel, docetaxel, or docetaxel/carboplatin:
Initial dose of 4 mg/kg as an intravenous infusion over 90 minutes then at 2 mg/kg as an intravenous infusion over 30 minutes weekly during chemotherapy for the first 12 weeks (paclitaxel or docetaxel) or 18 weeks (docetaxel/carboplatin).
One week following the last weekly dose of Trasturel, administer Trasturel at 6 mg/kg as an intravenous infusion over 30–90 minutes every three weeks.
As a single agent within three weeks following completion of multi-modality, anthracycline-based chemotherapy regimens:
- Initial dose at 8 mg/kg as an intravenous infusion over 90 minutes
- Subsequent doses at 6 mg/kg as an intravenous infusion over 30–90 minutes every three weeks.
- Extending adjuvant treatment beyond one year is not recommended.
Metastatic Treatment, Breast Cancer: Administer Trasturel, alone or in combination with paclitaxel, at an initial dose of 4 mg/kg as a 90-minute intravenous infusion followed by subsequent once weekly doses of 2 mg/kg as 30-minute intravenous infusions until disease progression.
Metastatic Gastric Cancer: Administer Trasturel at an initial dose of 8 mg/kg as a 90-minute intravenous infusion followed by subsequent doses of 6 mg/kg as an intravenous infusion over 30–90 minutes every three weeks until disease progression.
SIDE EFFECTS
Most common side effects are Nausea, Headache, Rash, Reduced blood platelets, Congestive cardiac failure, Insomnia (difficulty in sleeping), Infection, Upper respiratory tract infection, Nasopharyngitis, Fatigue, Fever, Anemia, Chills, Diarrhoea, Cough, Weight loss, Altered taste, Mucosal inflammation, Decreased white blood cell count (neutrophils), Stomatitis (Inflammation of the mouth)
PACK SIZE
Pack of 1 Vial
3S Corporation is a WHO GDSP approved & licensed Pharmaceutical stockist/wholesaler/distributor/exporter/importer of TrastuRel Trastuzumab 150mg & 440mg based in India - To buy TrastuRel Trastuzumab 150mg & 440mg or know its cost price contact us here.
We supply & sell TrastuRel Trastuzumab 150mg & 440mg for Reference Listed Drugs, Government Tenders, Shortage Lists, Emergency Imports, Name Patient Drugs, Comparator Drug Studies, Un-licensed Importation, clinical trial samples & Bio-Similars.