Description
Additional Information
Side Effects
Pack Size
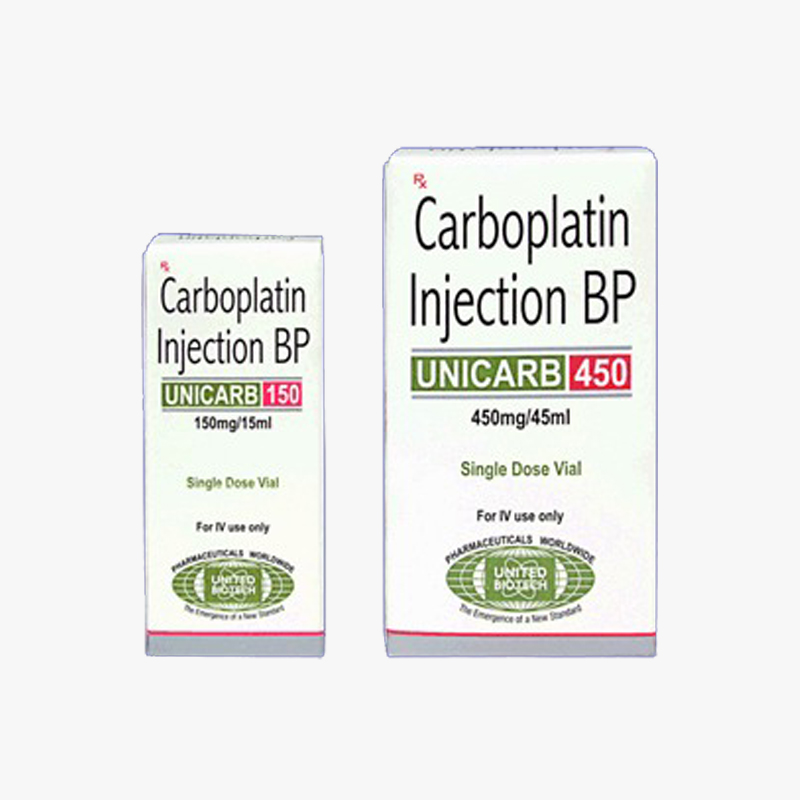
3S Corporation is a WHO GDSP approved & licensed Pharmaceutical stockist/wholesaler/distributor/exporter/importer of Lyophilized Carboplatin Injection 150mg & 450 mg based in India - To buy Lyophilized Carboplatin Injection 150mg & 450 mg or know its cost price contact us here.
We supply & sell Lyophilized Carboplatin Injection 150mg & 450 mg for Reference Listed Drugs, Government Tenders, Shortage Lists, Emergency Imports, Name Patient Drugs, Comparator Drug Studies, Un-licensed Importation, clinical trial samples & Bio-Similars.