DESCRIPTION
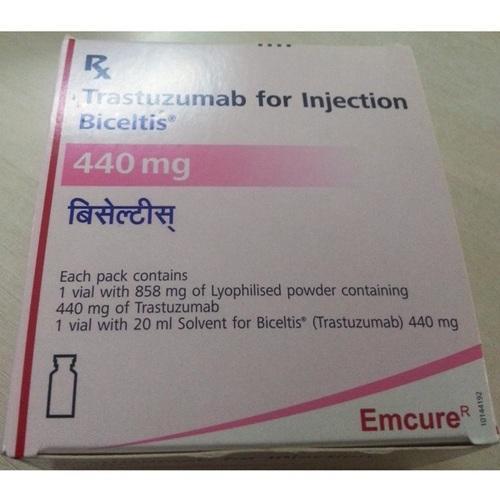
BICELTIS (Trastuzumab) is a monoclonal antibody that inhibits the growth of cancer cells and specifically designed to target HER2 receptors on breast cancer cells breast cancer cells which ultimately heals breast cancer. BICELTIS is a prescription drug.
Manufacturer : Emcure Pharmaceuticals Ltd
ADDITIONAL INFORMATION
Dosage :
Biceltis should be administered as intravenous infusion.
Loading Doses (Weekly):
- The recommended initial loading dose is 4 mg/kg body weight.
- Biceltis administered as a 90-minute intravenous infusion.
- Patients should be kept under observation for fever and chills or other infusion-associated symptoms.
- Interruption of the infusion may help control such symptoms.
- The infusion may be resumed when symptoms abate.
Subsequent Doses (Weekly):
- The recommended weekly dose of Biceltis is 2 mg/kg body weight.
- If the prior dose was well tolerated, the dose can be administered as a 30-minute infusion.
- Patients should be observed for fever and chills or other infusion-associated
Duration of Treatment: In clinical studies, patients with metastatic breast cancer or advanced gastric cancer were treated with Biceltis until progression of disease. Patients with early breast cancer should be treated for 1 year or until disease recurrence.
Strengths available : 150mg & 440mg
SIDE EFFECTS
Most common side effects are Nausea, Headache, Rash, Reduced blood platelets, Congestive cardiac failure, Insomnia (difficulty in sleeping), Infection, Upper respiratory tract infection, Nasopharyngitis, Fatigue, Fever, Anemia, Chills, Diarrhoea, Cough, Weight loss, Altered taste, Mucosal inflammation, Decreased white blood cell count (neutrophils), Stomatitis (Inflammation of the mouth).
PACK SIZE
Pack of 1 Vial
3S Corporation is a WHO GDSP approved & licensed Pharmaceutical stockist/wholesaler/distributor/exporter/importer of BICELTIS Trastuzumab 150mg & 440mg Injection based in India - To buy BICELTIS Trastuzumab 150mg & 440mg Injection or know its cost price contact us here.
We supply & sell BICELTIS Trastuzumab 150mg & 440mg Injection for Reference Listed Drugs, Government Tenders, Shortage Lists, Emergency Imports, Name Patient Drugs, Comparator Drug Studies, Un-licensed Importation, clinical trial samples & Bio-Similars.