Description
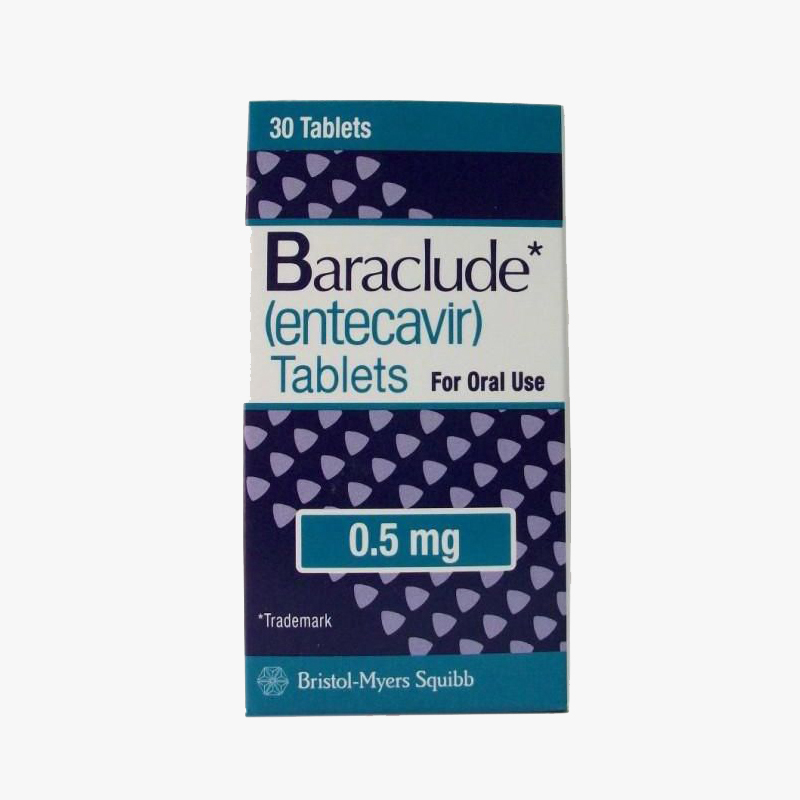
Baraclude is an oral antiviral drug used in the treatment of hepatitis B infection. It is also known as Entecavir. BARACLUDE works to reduce the amount of the hepatitis B virus (HBV) in the body, which may improve the condition of your liver.
Detailed Description:
Baraclude (entecavir) is a nucleoside analog with selectiveactivity against hepatitis B virus (HBV). Nucleoside analogsinhibit DNA synthesis in HBV infected cells, reducing viral loadand disease burden in infected patients. In animal studies, longterm administration of the drug was shown to reduce viral load toundetectable levels for 3-5 years.
Baraclude is specifically indicated for the treatment of chronicHBV infection in adults with evidence of active viral replicationand either evidence of persistent elevations in serumaminotransferases (ALT or AST) or histologically activedisease.
Baraclude is supplied as both an oral tablet and an oralsolution. The recommended dose for treatment-naïve adults andadolescents age 16 or older is 0.5 mg once daily on an emptystomach. The recommended dose is increased to 1.0 mg once daily forpatients with a history of hepatitis B viremia receiving lamivudineor with known lamivudine resistance mutations. For patients withrenal impairment (creatine clearance <50 mL/min), dosageadjustment may be necessary.
Additional Information
Side Effects
Pack Size
3S Corporation is a WHO GDSP approved & licensed Pharmaceutical stockist/wholesaler/distributor/exporter/importer of Baraclude 0.5MG based in India - To buy Baraclude 0.5MG or know its cost price contact us here.
We supply & sell Baraclude 0.5MG for Reference Listed Drugs, Government Tenders, Shortage Lists, Emergency Imports, Name Patient Drugs, Comparator Drug Studies, Un-licensed Importation, clinical trial samples & Bio-Similars.